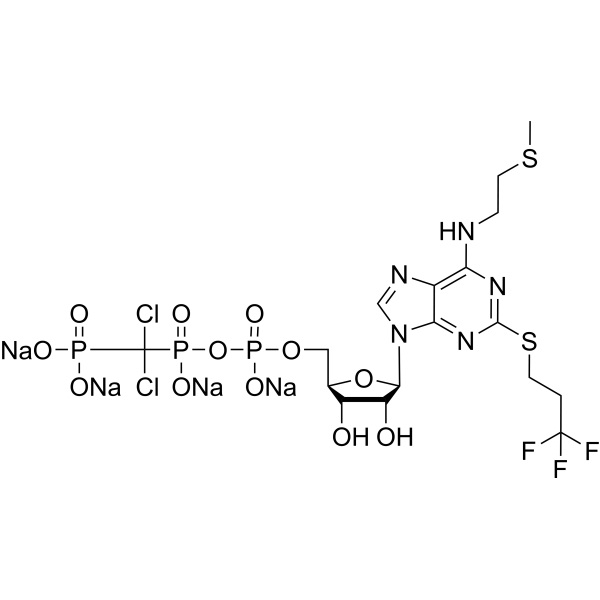
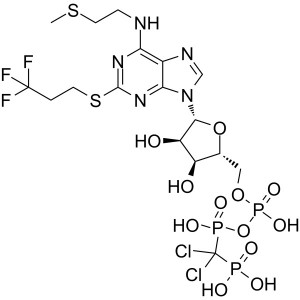
Cangrelor Tetrasodium CAS 163706-36-3 Purity >98.5% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Cangrelor Tetrasodium (CAS: 163706-36-3) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Cangrelor Tetrasodium, Please contact: alvin@ruifuchem.com
| Chemical Name | Cangrelor Tetrasodium |
| Synonyms | Cangrelor Tetrasodium Salt; AR-C 69931 Tetrasodium Salt; AR-C69931MX Tetrasodium Salt; N-[2-(Methylthio)ethyl]-2-[(3,3,3-Trifluoropropyl)thio] adenosine-5'-O-(β,γ-Dichloromethylene)triphosphate Tetrasodium Salt; N-[2-(Methylthio)ethyl]-2-[(3,3,3-Trifluoropropyl)thio]-,anhydride with P,P′-(Dichloromethylene)bis[phosphonic acid]-5′-Adenylic Acid Tetrasodium Salt |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 163706-36-3 |
| Molecular Formula | C17H21Cl2F3N5O12P3S2·Na4 |
| Molecular Weight | 864.29 |
| Sensitive | Light Sensitive, Moisture Sensitive |
| Water Solubility | H2O: 2 mg/mL, Clear |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Brand | Ruifu Chemical |
| Items | Specifications |
| Appearance | White to Beige Powder |
| Purity / Analysis Method | >98.5% (HPLC) |
| Water by Karl Fischer | <13.50% |
| Sodium (Na) | 10.32~10.96% |
| 1H NMR Spectrum | Consistent with Structure |
| LC-MS | Consistent with Structure |
| Conclusion | The product has been tested and complies with the given specifications |
None of the products will be supplied to countries in which this could be in conflict with the existing patents. However the final responsibility lies with the Buyer.
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Cangrelor Tetrasodium (CAS: 163706-36-3) is a direct purinergic platelet receptor (P2Y12) inhibitor that blocks ADP-induced platelet activation and aggregation. The drug, which was developed by The Medicine Company, binds reversibly to the P2Y12 receptor, preventing further signaling and platelet activation. Cangrelor, which was approved in June 2015 by the FDA, is indicated as an adjunct to percutaneous coronary intervention for reducing the risk of periprocedural myocardial infarction, repeat coronary revascularization, and stent thrombosis in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor. The most common side effect observed with the drug was bleeding.
Cangrelor is a recently approved intravenous P2Y12 antagonist with a rapid onset of action (2–3 minutes) and recovery of platelet function after discontinuation. In the CHAMPION-PHOENIX trial, compared with patients treated with oral clopidogrel at the time of or immediately after PCI, cangrelor-treated patients had a lower risk of recurrent MI or stent thrombosis at the cost of an increase in minor but not major bleeding. As a result, in patients who were not adequately pretreated with clopidogrel prior to PCI, cangrelor may provide sufficient platelet inhibition to conduct PCI safely.