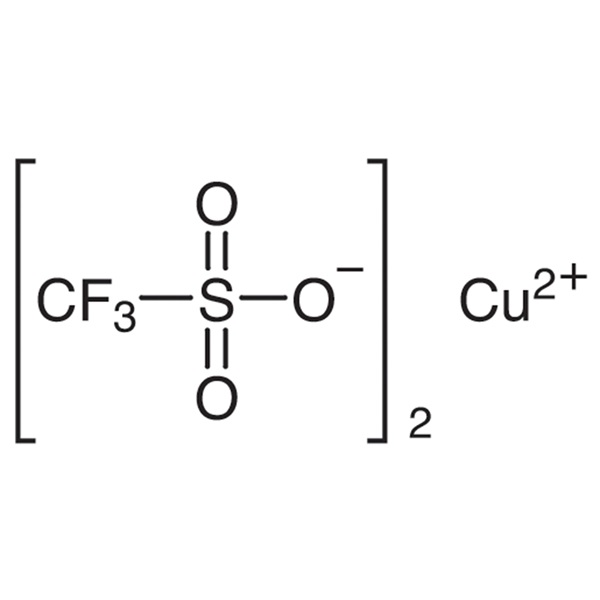
Copper(II) Trifluoromethanesulfonate CAS 34946-82-2 Purity >98.0% (Titration) Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Copper(II) Trifluoromethanesulfonate (CAS: 34946-82-2) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com
| Chemical Name | Copper(II) Trifluoromethanesulfonate |
| Synonyms | Copper Trifluoromethanesulfonate; Copper(II) Triflate; Trifluoromethanesulfonic Acid Copper(II) Salt; Cu(OTf)2 |
| CAS Number | 34946-82-2 |
| CAT Number | RF-PI2078 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C2CuF6O6S2 |
| Molecular Weight | 361.67 |
| Water Solubility | Soluble in Water |
| Sensitivity | Hygroscopic |
| Melting Point | ≥300℃ |
| Storage Temp. | Inert Atmosphere, Room Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | Off-White to Light Blue Solid |
| Purity / Analysis Method | >98.0% (Titration) |
| Moisture | <0.20% |
| Carbon by Elemental Analysis | 6.0~7.1% |
| Oxygen by Elemental Analysis | 25.5~26.9% |
| ICP | Confirms Cu Components Confirmed |
| Infrared Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
Package: 25kg/Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Copper(II) Trifluoromethanesulfonate (CAS: 34946-82-2) is usually used as catalyst for Mannich condensation, Annulative amination, Friedel-Crafts reaction, Henry reaction, Hypervalent iodine reagent-mediated preparation of carbazoles, Intramolecular oxidative C-N bond formation for the synthesis of carbazoles, the efficient addition of trimethylsilyl cyanide to carbonyl compounds. Ring-Opening of epoxides and aziridines. Asymmetric conjugate addition of organozinc reagents to α,β-unsaturated ketones. Electrophilic addition of olefins. Asymmetric aziridination of olefins. Asymmetric cycloadditions and aldol condensations. Asymmetric Kharasch oxidation. Asymmetric Michael addition of enamides. Asymmetric O-H or O-R insertion reactions. Enantioselective intramolecular aminooxygenation of alkenes. Enantioselective addition of dialkylzinc reagents to N-acylpyridinium salts. Pd-catalyzed C-H functionalizations of oximes with arylboronic acids. Used as a Lewis acid in the Nazarov cyclization. Catalyst in the diacetoxylation olefins. Catalyst in the meta-selective direct arylation of α-aryl carbonyl compounds. Catalyst in the three-component coupling of amines, aldehydes, and alkynes.
-
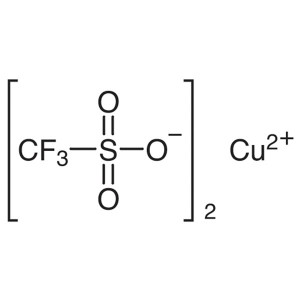
Copper(II) Trifluoromethanesulfonate CAS 34946-...
-
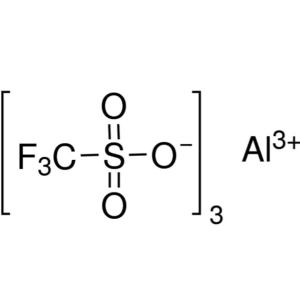
Aluminum Trifluoromethanesulfonate CAS 74974-61...
-
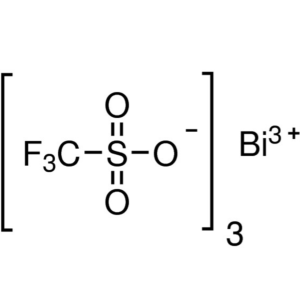
Bismuth(III) Trifluoromethanesulfonate CAS 8818...
-
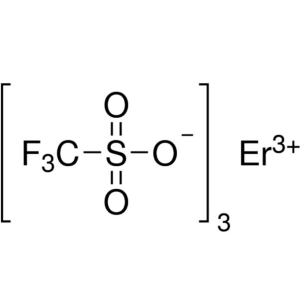
Erbium(III) Trifluoromethanesulfonate CAS 13917...
-
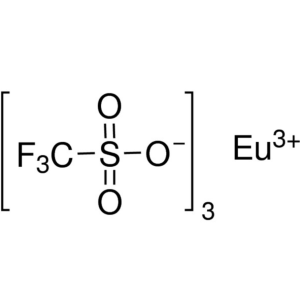
Europium(III) Trifluoromethanesulfonate CAS 520...
-
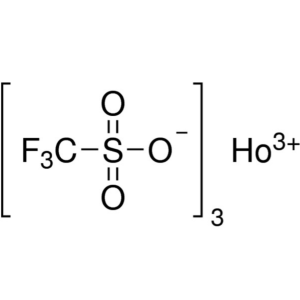
Holmium(III) Trifluoromethanesulfonate CAS 1391...
-
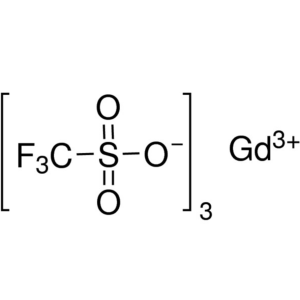
Gadolinium(III) Trifluoromethanesulfonate CAS 5...
-
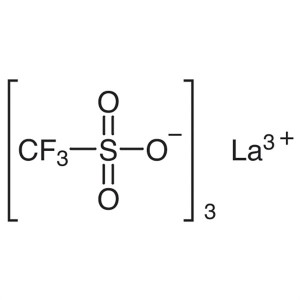
Lanthanum(III) Trifluoromethanesulfonate CAS 52...
-
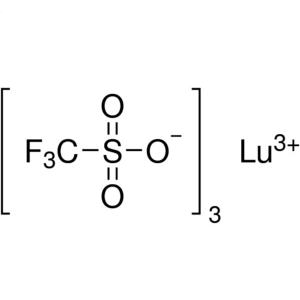
Lutetium(III) Trifluoromethanesulfonate CAS 126...
-
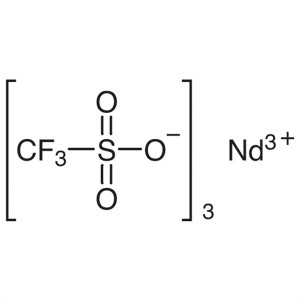
Neodymium(III) Trifluoromethanesulfonate CAS 34...
-
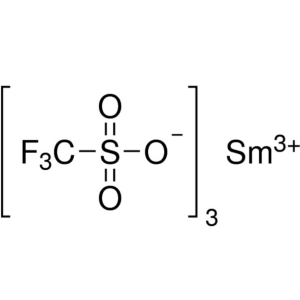
Samarium(III) Trifluoromethanesulfonate CAS 520...
-
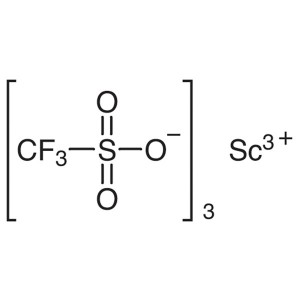
Scandium(III) Trifluoromethanesulfonate CAS 144...
-
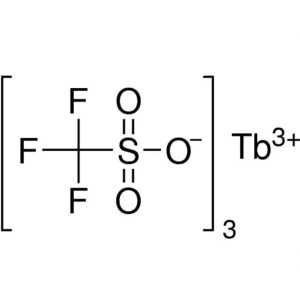
Terbium(III) Trifluoromethanesulfonate CAS 1489...
-
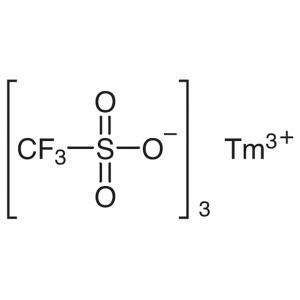
Thulium(III) Trifluoromethanesulfonate CAS 1414...
-
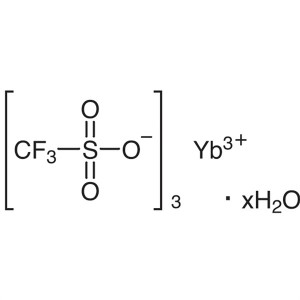
Ytterbium(III) Trifluoromethanesulfonate Hydrat...
-
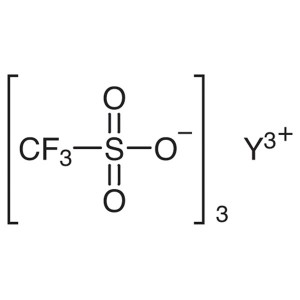
Yttrium(III) Trifluoromethanesulfonate CAS 5209...